Home » Cymbalta: Dual the Reuptake, Triple the Hype
Cymbalta: Dual the Reuptake, Triple the Hype
January 1, 2004
From The Carlat Psychiatry Report
Daniel Carlat, MD
Dr. Carlat has disclosed that he has no significant relationships with or financial interests in any commercial companies pertaining to this educational activity.
We have some good news and we have some bad news. First, the good news: Cymbalta (generic name: duloxetine) is an effective dual reuptake antidepressant with a good safety profile. Now, the bad news: it appears to have no advantages over existing antidepressants.
Of course, this is not exactly the impression you'll be getting from Lilly-sponsored articles and CME events over the next few months leading up to its FDA approval. You'll be hearing a lot about its "high" remission rate, and about its unparalleled performance in relieving the somatic symptoms of depression.
These are not lies, exactly. But when it comes to new drug launches into multi-billion dollar markets, truth is an elusive commodity. And the launch of Cymbalta is a textbook example of this phenomenon.
Cymbalta's effectiveness for painful symptoms of depression. You may have noticed that much of Cymbalta's pre-release promotional literature focuses on the "emotional and physical symptoms of depression." The patients enrolled in the Cymbalta trials must have been pretty surprised when researchers started asking questions about back pain, shoulder pain, and headache, but a fair number of them endorsed such symptoms, and Cymbalta tended to ease these pains better than placebo.
Of course, the scale Lilly used, the VAS (Visual Analog Scale Measure of Pain Severity), has never been used in antidepressant trials before. We assume competing antidepressants would treat aches and pains just as well. The argument against this assumption is that those antidepressants with the best data for pain management-the tertiary tricyclics (amitriptyline and imipramine)-are both dual reuptake inhibitors, like Cymbalta. Nonetheless, unlike the compelling studies of tricyclics for pain management (Fishbain, Ann Med 2000; 32:305-316), there are as yet no published studies endorsing Cymbalta's analgesic effectiveness.
Thus, at this point Cymbalta's "painful symptom" literature comes across as a niche-seeking marketing ploy. We await equivalent VAS data from other antidepressants before passing final judgment.
Cymbalta's "high" remission rates. Read the following quote from the abstract of a recent article by Lillyfunded researchers: "Probabilities of remission >55% were observed in two of the studies, while in a third study the probability of remission with duloxetine treatment was nearly three times that observed with placebo (44% versus 16%)" (Nemeroff et al, Psychopharmacology Bulletin, 2002;36:106- 132).
Sounds pretty impressive. Especially when you compare these numbers with the 45% Effexor remission rate trumpeted by Wyeth as reported in the famous "pooled analysis" paper by Michael Thase (Thase, Brit J Psychiatry, 2001;178:234-241).
However, in a breathtaking display of comparing apples with oranges, Lilly has brandished a completely new way of analyzing efficacy results, a method that boosts its remission numbers by 25-30%! We are not making this up. For proof, look at the table below.
Table: Published Controlled Trials of Cymbalta (or, Fun with Statistics)
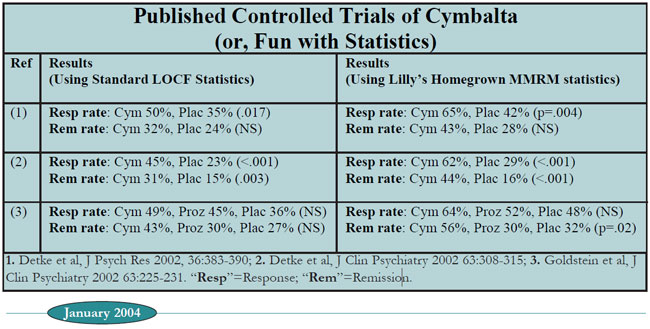
The first column lists the references to the three published placebocontrolled trials of Cymbalta as of January 2004.
The second column lists response and remission rates of Cymbalta vs. placebo, using the statistical method that has been used for all recent FDAregistered antidepressants trials until Cymbalta. This is the "LOCF" (last observation carried forward) method, in which the last depression scale score obtained from a patient (ie, the "last observation") is used in the final analysis, even if that patient dropped out early in the study.
Using these tried and true statistics, Cymbalta looks like an effective antidepressant, but with numbers that are hardly spectacular: We see response rates of 45-50%, and remission rates of 31-43%, with one of the three studies showing no statistical difference from placebo.
The third column is where things get interesting. Instead of using the LOCF method, these numbers are calculated using the MMRM ("mixedeffects model repeated-measures") analysis. This method was adapted by Eli Lilly statisticians from an established method called “repeated-measures analysis” in order to more accurately account for the effects of the high patient drop-out rate in clinical trials. We won't torture you with details, but interested readers should peruse an excellent article by Lilly statistician Craig Mallinckrodt et al (Biol Psychiatry, 2003;53:754- 760). While TCR's statistical consultants like this method, it is far from becoming the industry's standard.
The bottom line is that this new technique generally results in drug effects separating much more robustly from placebo than the old LOCF method.
And this is certainly true for Cymbalta. Otherwise paltry response rates sparkle under the MMRM spotlight, jumping up into the 60-65% range. Remission rates leap into the stratosphere as well, to as high as 56% in ref. 3.
The statistics are sound, but the presentation is devious. A more ethical approach would be to present only the LOCF results in the abstracts, and to present the unorthodox MMRM results in a separate, secondary section within the body of the paper.
But then Cymbalta would come across as just another modestly effective antidepressant-exactly what it is.
TCR VERDICT Cymbalta: Average Antidepressant; Spectacular Hype
General PsychiatryOf course, this is not exactly the impression you'll be getting from Lilly-sponsored articles and CME events over the next few months leading up to its FDA approval. You'll be hearing a lot about its "high" remission rate, and about its unparalleled performance in relieving the somatic symptoms of depression.
These are not lies, exactly. But when it comes to new drug launches into multi-billion dollar markets, truth is an elusive commodity. And the launch of Cymbalta is a textbook example of this phenomenon.
A new Eli Lilly statistic makes all of Cymbalta’s numbers look grand!
Cymbalta's effectiveness for painful symptoms of depression. You may have noticed that much of Cymbalta's pre-release promotional literature focuses on the "emotional and physical symptoms of depression." The patients enrolled in the Cymbalta trials must have been pretty surprised when researchers started asking questions about back pain, shoulder pain, and headache, but a fair number of them endorsed such symptoms, and Cymbalta tended to ease these pains better than placebo.
Of course, the scale Lilly used, the VAS (Visual Analog Scale Measure of Pain Severity), has never been used in antidepressant trials before. We assume competing antidepressants would treat aches and pains just as well. The argument against this assumption is that those antidepressants with the best data for pain management-the tertiary tricyclics (amitriptyline and imipramine)-are both dual reuptake inhibitors, like Cymbalta. Nonetheless, unlike the compelling studies of tricyclics for pain management (Fishbain, Ann Med 2000; 32:305-316), there are as yet no published studies endorsing Cymbalta's analgesic effectiveness.
Thus, at this point Cymbalta's "painful symptom" literature comes across as a niche-seeking marketing ploy. We await equivalent VAS data from other antidepressants before passing final judgment.
Cymbalta's "high" remission rates. Read the following quote from the abstract of a recent article by Lillyfunded researchers: "Probabilities of remission >55% were observed in two of the studies, while in a third study the probability of remission with duloxetine treatment was nearly three times that observed with placebo (44% versus 16%)" (Nemeroff et al, Psychopharmacology Bulletin, 2002;36:106- 132).
Sounds pretty impressive. Especially when you compare these numbers with the 45% Effexor remission rate trumpeted by Wyeth as reported in the famous "pooled analysis" paper by Michael Thase (Thase, Brit J Psychiatry, 2001;178:234-241).
However, in a breathtaking display of comparing apples with oranges, Lilly has brandished a completely new way of analyzing efficacy results, a method that boosts its remission numbers by 25-30%! We are not making this up. For proof, look at the table below.
Table: Published Controlled Trials of Cymbalta (or, Fun with Statistics)

The first column lists the references to the three published placebocontrolled trials of Cymbalta as of January 2004.
The second column lists response and remission rates of Cymbalta vs. placebo, using the statistical method that has been used for all recent FDAregistered antidepressants trials until Cymbalta. This is the "LOCF" (last observation carried forward) method, in which the last depression scale score obtained from a patient (ie, the "last observation") is used in the final analysis, even if that patient dropped out early in the study.
Using these tried and true statistics, Cymbalta looks like an effective antidepressant, but with numbers that are hardly spectacular: We see response rates of 45-50%, and remission rates of 31-43%, with one of the three studies showing no statistical difference from placebo.
The third column is where things get interesting. Instead of using the LOCF method, these numbers are calculated using the MMRM ("mixedeffects model repeated-measures") analysis. This method was adapted by Eli Lilly statisticians from an established method called “repeated-measures analysis” in order to more accurately account for the effects of the high patient drop-out rate in clinical trials. We won't torture you with details, but interested readers should peruse an excellent article by Lilly statistician Craig Mallinckrodt et al (Biol Psychiatry, 2003;53:754- 760). While TCR's statistical consultants like this method, it is far from becoming the industry's standard.
The bottom line is that this new technique generally results in drug effects separating much more robustly from placebo than the old LOCF method.
And this is certainly true for Cymbalta. Otherwise paltry response rates sparkle under the MMRM spotlight, jumping up into the 60-65% range. Remission rates leap into the stratosphere as well, to as high as 56% in ref. 3.
The statistics are sound, but the presentation is devious. A more ethical approach would be to present only the LOCF results in the abstracts, and to present the unorthodox MMRM results in a separate, secondary section within the body of the paper.
But then Cymbalta would come across as just another modestly effective antidepressant-exactly what it is.
TCR VERDICT Cymbalta: Average Antidepressant; Spectacular Hype
KEYWORDS antidepressants

Issue Date: January 1, 2004
Table Of Contents
Recommended
Newsletters
Please see our Terms and Conditions, Privacy Policy, Subscription Agreement, Use of Cookies, and Hardware/Software Requirements to view our website.
© 2026 Carlat Publishing, LLC and Affiliates, All Rights Reserved.


_-The-Breakthrough-Antipsychotic-That-Could-Change-Everything.webp?t=1729528747)



