Home » Intuniv: Exorbitant, Sedating, and Second-Line
Intuniv: Exorbitant, Sedating, and Second-Line
November 1, 2014
From The Carlat Child Psychiatry Report
Talia Puzantian, PharmD, BCPP
Guanfacine, a drug originally used to treat hypertension, has been reformulated and now has new life as a medication to treat ADHD in children.
An alpha-2A agonist, this medication was initially marketed as Tenex (now available as a generic) for treating hypertension. However, centrally-acting antihypertensives such as guanfacine fell out of favor as newer and less sedating antihypertensives came on the scene.
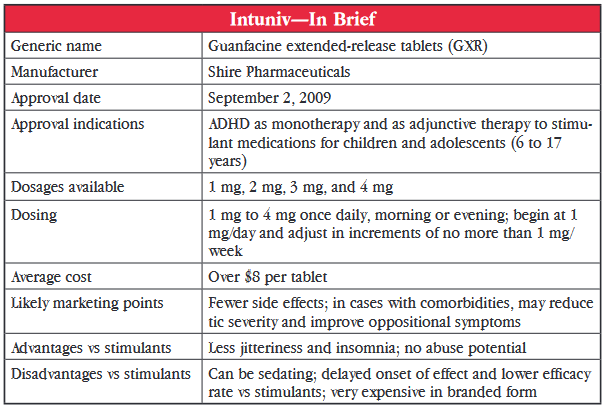
In more recent years, guanfacine use has had a resurgence, but this time in children with ADHD. Small pilot studies conducted as far back as 20 years ago showed it had promise in this group. And, in 2009, an extended-release formulation of guanfacine (GXR) marketed as Intuniv, was approved by the US Food and Drug Administration (FDA) for 6 to 17 year olds with ADHD, either as monotherapy or as an adjunct to stimulants. Just to clarify, guanfacine immediate-release is available as a generic, but GRX is available as brand-name Intuniv only.
What Studies Show
The FDA approval was based on results from two controlled clinical trials comparing GXR (1 mg to 4 mg/day) to placebo in a total of 669 children and adolescents. GXR was significantly more effective for inattentive and hyperactive-impulsive symptoms than placebo at all doses. But, when the data for adolescents aged 13 to 17 years were separated, no significant difference was noted in that group. A third preclinical study assessed the efficacy of adjunctive GXR in 461 children with suboptimal response to stimulants. Significant improvement was seen in all GXR groups (1 mg to 4 mg/day) over placebo groups, this time including the adolescents.
Unfortunately, there have been no published trials comparing guanfacine to other approved medications for ADHD although a search of clinical trial registries finds two unpublished head-to-head studies, one versus methylphenidate (NCT00429273) and the other versus atomoxetine (NCT01244490). Until we can see results of those studies, the best we can do to determine guanfacine’s place in therapy is to take a look at effect sizes. Effect size is a measure that describes the magnitude of treatment effect. Studies of GXR have shown effect sizes between 0.43 and 0.86, depending on dose used. This is a lower effect size than those reported for stimulants (0.8 to1.2) and about the same as for atomoxetine (0.59 to 0.64). By comparison, GXR used as an adjunct to stimulants resulted in a lower effect size (about 0.4).
Two studies examined the use of guanfacine in ADHD patients with comorbidities. One controlled trial showed significant improvement, compared to placebo, in reducing tic severity and another showed significant improvement in oppositional symptoms. This isn’t really enough to make a hearty statement but it would be nice to see if more studies would help determine which types of patients may be the best candidates for guanfacine therapy.
A marked difference between guanfacine and stimulants are the side effects. Stimulants commonly cause insomnia, anorexia, and tachycardia. The most common side effects of guanfacine were fatigue and somnolence, which were reported by as many as 33% to 44% of patients. Up to 18% of patients discontinued treatment because of these effects. Although sedation was very common and dose-related in the monotherapy trials, it occurred less frequently when guanfacine was used as an adjunct to stimulant. Patients also reported headache, nausea, and dry mouth with guanfacine but these tended to be mild to moderate. Dose-related decreases in blood pressure can occur rarely and warrant caution particularly when treatment is initiated or when dose is increased.
The pharmacokinetics do suggest that substituting on a mg-to-mg basis is not recommended but an adjusted dosing substitution would be appropriate. The dosing recommendation for Intuniv is to start at 1 mg/day and to increase by 1 mg/day weekly to a maximum of 4 mg/day. The immediate-release generic can be initiated at 0.5–1 mg QHS (at bedtime) and increased by 1 mg/day weekly to a maximum of 2 mg to 4 mg/day, depending on the patient’s weight (60–90 pounds: 2 mg/day; 90–99 pounds: 3 mg/day; >99 pounds: 4 mg/day).
The dose of immediate-release formulation is usually divided to minimize hypotension or orthostasis, which occur only rarely. It’s interesting to note that the half-lives of the two formulations do not differ by much, unlike other medications with extended-release formulations. In adults, Intuniv has a half-life (t ½) of 18 hours while immediate-release has a half-life (t ½) of 16 hours (it’s about 13–14 hours in children).
Dr. Carlat’s Verdict: Is Intuniv something new? Yes, this is something new for ADHD; it’s not simply another stimulant with the standard potential problems of substance abuse, insomnia, and poor appetite. It’s a sedating agent that could be beneficial for some patients. It seems to be less effective overall than stimulants. But, for certain types of children, particularly younger children or those with certain comorbidities, it may be a decent second-line option or an add-on to stimulants. The tenfold difference in price between the extended-release and immediate-release products is extremely difficult to justify, so go with the cheaper, generic, immediate-release first.
Child PsychiatryAn alpha-2A agonist, this medication was initially marketed as Tenex (now available as a generic) for treating hypertension. However, centrally-acting antihypertensives such as guanfacine fell out of favor as newer and less sedating antihypertensives came on the scene.
In more recent years, guanfacine use has had a resurgence, but this time in children with ADHD. Small pilot studies conducted as far back as 20 years ago showed it had promise in this group. And, in 2009, an extended-release formulation of guanfacine (GXR) marketed as Intuniv, was approved by the US Food and Drug Administration (FDA) for 6 to 17 year olds with ADHD, either as monotherapy or as an adjunct to stimulants. Just to clarify, guanfacine immediate-release is available as a generic, but GRX is available as brand-name Intuniv only.
What Studies Show
The FDA approval was based on results from two controlled clinical trials comparing GXR (1 mg to 4 mg/day) to placebo in a total of 669 children and adolescents. GXR was significantly more effective for inattentive and hyperactive-impulsive symptoms than placebo at all doses. But, when the data for adolescents aged 13 to 17 years were separated, no significant difference was noted in that group. A third preclinical study assessed the efficacy of adjunctive GXR in 461 children with suboptimal response to stimulants. Significant improvement was seen in all GXR groups (1 mg to 4 mg/day) over placebo groups, this time including the adolescents.
Unfortunately, there have been no published trials comparing guanfacine to other approved medications for ADHD although a search of clinical trial registries finds two unpublished head-to-head studies, one versus methylphenidate (NCT00429273) and the other versus atomoxetine (NCT01244490). Until we can see results of those studies, the best we can do to determine guanfacine’s place in therapy is to take a look at effect sizes. Effect size is a measure that describes the magnitude of treatment effect. Studies of GXR have shown effect sizes between 0.43 and 0.86, depending on dose used. This is a lower effect size than those reported for stimulants (0.8 to1.2) and about the same as for atomoxetine (0.59 to 0.64). By comparison, GXR used as an adjunct to stimulants resulted in a lower effect size (about 0.4).
Two studies examined the use of guanfacine in ADHD patients with comorbidities. One controlled trial showed significant improvement, compared to placebo, in reducing tic severity and another showed significant improvement in oppositional symptoms. This isn’t really enough to make a hearty statement but it would be nice to see if more studies would help determine which types of patients may be the best candidates for guanfacine therapy.
A marked difference between guanfacine and stimulants are the side effects. Stimulants commonly cause insomnia, anorexia, and tachycardia. The most common side effects of guanfacine were fatigue and somnolence, which were reported by as many as 33% to 44% of patients. Up to 18% of patients discontinued treatment because of these effects. Although sedation was very common and dose-related in the monotherapy trials, it occurred less frequently when guanfacine was used as an adjunct to stimulant. Patients also reported headache, nausea, and dry mouth with guanfacine but these tended to be mild to moderate. Dose-related decreases in blood pressure can occur rarely and warrant caution particularly when treatment is initiated or when dose is increased.
Price and Pharmacokinetics
The pharmacokinetics do suggest that substituting on a mg-to-mg basis is not recommended but an adjusted dosing substitution would be appropriate. The dosing recommendation for Intuniv is to start at 1 mg/day and to increase by 1 mg/day weekly to a maximum of 4 mg/day. The immediate-release generic can be initiated at 0.5–1 mg QHS (at bedtime) and increased by 1 mg/day weekly to a maximum of 2 mg to 4 mg/day, depending on the patient’s weight (60–90 pounds: 2 mg/day; 90–99 pounds: 3 mg/day; >99 pounds: 4 mg/day).
The dose of immediate-release formulation is usually divided to minimize hypotension or orthostasis, which occur only rarely. It’s interesting to note that the half-lives of the two formulations do not differ by much, unlike other medications with extended-release formulations. In adults, Intuniv has a half-life (t ½) of 18 hours while immediate-release has a half-life (t ½) of 16 hours (it’s about 13–14 hours in children).
Dr. Carlat’s Verdict: Is Intuniv something new? Yes, this is something new for ADHD; it’s not simply another stimulant with the standard potential problems of substance abuse, insomnia, and poor appetite. It’s a sedating agent that could be beneficial for some patients. It seems to be less effective overall than stimulants. But, for certain types of children, particularly younger children or those with certain comorbidities, it may be a decent second-line option or an add-on to stimulants. The tenfold difference in price between the extended-release and immediate-release products is extremely difficult to justify, so go with the cheaper, generic, immediate-release first.
KEYWORDS adhd child-psychiatry

Issue Date: November 1, 2014
Table Of Contents
Recommended
Newsletters
Please see our Terms and Conditions, Privacy Policy, Subscription Agreement, Use of Cookies, and Hardware/Software Requirements to view our website.
© 2026 Carlat Publishing, LLC and Affiliates, All Rights Reserved.


_-The-Breakthrough-Antipsychotic-That-Could-Change-Everything.webp?t=1729528747)



