A New Proposal for SSRI Withdrawal
Drs. Sazima and Aiken have disclosed that they have no relevant financial or other interests in any commercial companies pertaining to this educational activity.
Review of: Horowitz MA and Taylor D, Lancet Psychiatry, March 2019, published online
Type of Study: Literature review and clinical guideline
Withdrawal problems rank among patients’ top concerns with antidepressants. They include insomnia, flu-like symptoms, irritability, distractibility, and unusual sensory experiences such as “brain zaps.” To avoid this syndrome, most clinicians taper antidepressants linearly, lowering the dose by a fixed amount at a regular rate. For example, a typical linear taper of citalopram 30 mg daily might proceed like this: citalopram 20 mg daily for 1 week, 10 mg daily for 1 week, then stop. A new study suggests we might need to rethink that practice.
The authors examined clinical and biological research on tapers of serotonergic antidepressants. Their review included six major clinical trials examining SSRI and SNRI tapers, covering a total of 1,687 patients. The clinical outcomes on linear tapers were problematic. In one study, 60% of patients required 4 months or more to taper. In another, patients had particular difficulty at the final tail of the taper. They stalled when the taper reached half of the minimum therapeutic dose for depression (eg, when citalopram reached 10 mg daily). Only 37% were able to complete the taper beyond that point.
To better understand these problems, the authors looked at PET scan data. In line with the clinical findings, there was a dramatic fall in serotonin transporter inhibition when linear tapers reached half of the minimum therapeutic dose for depression. Inhibition of the serotonin transporter is the principal mechanism of SSRIs, and changes in that inhibition are thought to contribute to serotonergic withdrawal symptoms.
The authors proposed a new tapering strategy to prevent this dramatic fall: the “hyperbolic taper.” In this taper, the SSRI is lowered with a long tail of incrementally smaller dose reductions. With this strategy, the serotonin inhibition would fall linearly as the SSRI is tapered at an increasingly slower rate. Theoretically, this “hyperbolic taper” could prevent serotonin withdrawal symptoms. The authors recommend the same strategy with fluoxetine, although the risk of withdrawal problems is lower with this SSRI because of its long half-life.
TCPR’S TAKE
Tapers with a long tail have been used successfully with benzodiazepines, and the clinical and biological data presented here suggest they may be preferable with serotonergic antidepressants as well. Although the idea lacks clinical proof, so does current practice, and a “hyperbolic taper” is worth trying in patients who have trouble with serotonin withdrawal.
The Hyperbolic Taper for SSRIs
-
Lower to the minimum dose
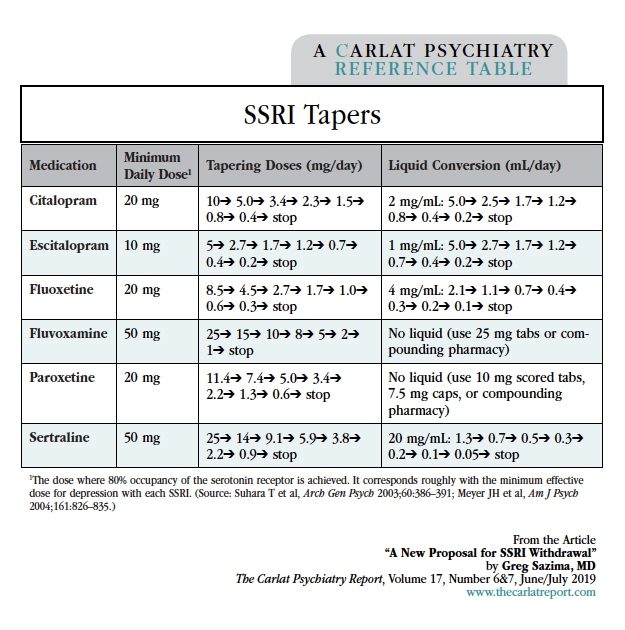
Reduce the dose to the minimum suggested in the table on this page if not already there (eg, citalopram 20 mg). The dose can be reduced linearly at this stage because the main risk is depressive relapse, not serotonin withdrawal. Consider the patient’s history, including risk of relapse and prior response to lower doses, in determining the rate of the taper. Lowering every 1–2 weeks is reasonable for most patients.
-
Assess baseline symptoms
Check if the patient is having any SSRI withdrawal symptoms at baseline (see www.thecarlatreport.com/serotonin).
-
Lower for one month and reassess
Now move to the first tapering dose in the table (eg, citalopram 10 mg). After a month, reassess for SSRI withdrawal symptoms.
-
Start the long-tail taper
The doses for each step of the final taper are listed in the table (eg, citalopram 5 mg, then 3.4 mg). How quickly you go down at each step depends on how sensitive the patient is to withdrawal; your assessment of symptoms at baseline and one month later will give you a sense of this. At a minimum, allow 1 week between each step; 2 weeks is a rough average, and sensitive patients may require 6 weeks between each step.
Table: SSRI Tapers

Newsletters
Please see our Terms and Conditions, Privacy Policy, Subscription Agreement, Use of Cookies, and Hardware/Software Requirements to view our website.
© 2026 Carlat Publishing, LLC and Affiliates, All Rights Reserved.


_-The-Breakthrough-Antipsychotic-That-Could-Change-Everything.webp?t=1729528747)



