Brexpiprazole’s New Claims

Kate Travis, MD. Attending Psychiatrist, Houston Methodist, Department of Psychiatry and Behavioral Health. Volunteer Adjunct Assistant Professor, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences.
Dr. Travis has no financial relationships with companies related to this material.
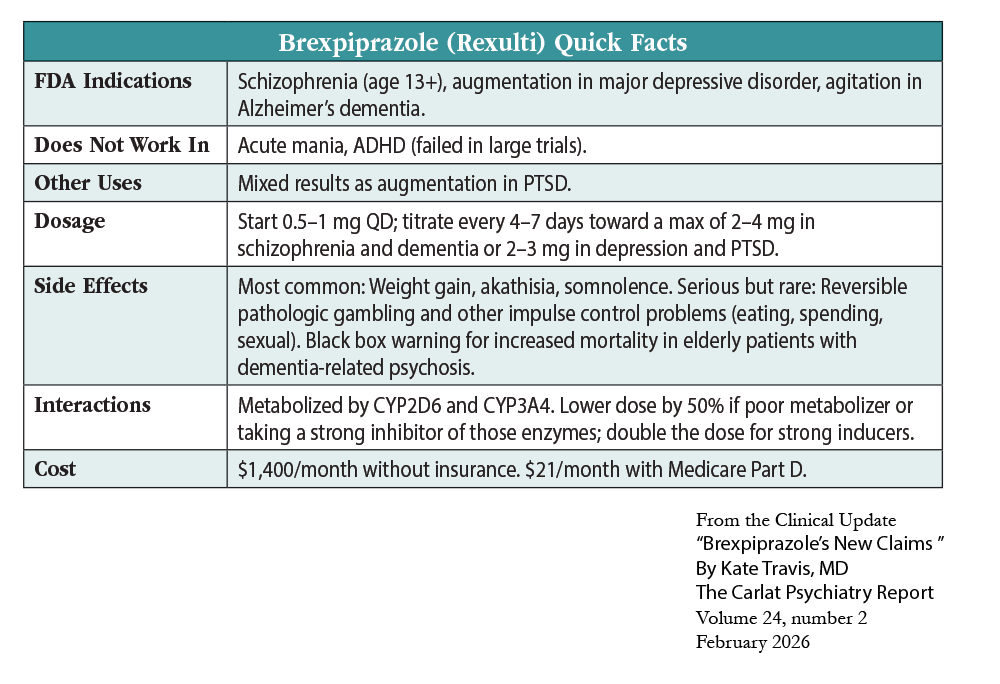
Brexpiprazole (Rexulti) has emerged as a leading “third-generation” antipsychotic with a widening range of applications. This generation is marked by low dopamine (D2) blockade and includes aripiprazole, cariprazine, and lumateperone. Brexpiprazole entered the market in 2015 and received its first FDA approvals for schizophrenia (age 13 and up) and as an adjunctive therapy for adults with major depressive disorder. In May 2023, it earned its third FDA approval for agitation associated with dementia due to Alzheimer’s disease. In this article, I’ll explore the evidence behind these expanding claims, starting with its failure in PTSD.
PTSD
The FDA recently rejected brexpiprazole as a combination therapy with sertraline for PTSD. Only one of the two large trials was positive, and that trial’s conditions did not reflect the real world. Psychiatrist Mark Zimmerman estimates that only 5% of outpatients with PTSD would have met the strict inclusion criteria. Psychiatrist Michael Sikorav highlighted additional problems with the trials:
- Mixed results: The positive trial was only positive in women.
- Biased sample: Patients who were on psychotropics before the trials had to stop their medication. Those who stopped improved more with the combo pill than those who were not on medication, suggesting the active treatment may have a withdrawal syndrome.
- High dropout rates: Dropout rates ranged from 30% to 40%.
While other antipsychotics have positive augmentation trials in PTSD,† brexpiprazole may have a unique role. In animal models, it prevented consolidation of traumatic memories, possibly due to effects on the hippocampus or its partial agonist activity at serotonin 5-HT1A and D2 receptors (Ducourneau EG et al, Mol Psychiatry 2021;26(7):3018–3033).
Brexpiprazole was dosed at 2–3 mg, with sertraline at 150 mg, in the PTSD trials. Brexpiprazole was surprisingly well tolerated, with similar rates of dropouts due to adverse effects as placebo. However, short-term trials are likely to miss many of the serious risks of antipsychotics (Brubaker M et al, Neuropsychiatr Dis Treat 2025;21:421–436).
Carlat Verdict: We don’t recommend antipsychotics first line in PTSD, but brexpiprazole is a reasonable option if you need to augment after trying a selective serotonin reuptake inhibitor and psychotherapy. To prevent long-term risks, attempt to taper the antipsychotic off after six to nine months of recovery.
Agitation related to dementia in Alzheimer’s disease
Agitation is one of the most stressful, complex, and expensive challenges in dementia associated with Alzheimer’s disease. In May 2023, brexpiprazole became the first medication to be approved by the FDA for this indication. The lack of FDA-approved predecessors was not for lack of trying. For decades, clinicians and researchers have tested various antipsychotics and medications to alleviate agitation. Unfortunately, the benefits of these treatments have been meager and the risks substantial, including a nearly two-fold increased risk of death in elderly patients with dementia exposed to antipsychotics—which earned antipsychotics their black box warning in 2015.
Two pharmaceutical companies, Lundbeck and Otsuka, secured brexpiprazole’s FDA approval for agitation related to Alzheimer’s dementia. The FDA initially doubted the efficacy shown in the first pair of placebo-controlled trials and requested a third study, which convinced 9 of 10 FDA committee members to vote in favor of approval. The studies included 1,048 patients with Alzheimer’s disease and agitation. Each trial lasted 12 weeks and followed a randomized, double-blind, placebo-controlled design. Brexpiprazole doses were titrated to target doses of 0.5, 1, 2, or 3 mg, with positive results for the 2–3 mg range but not for the lower doses.
While the results were statistically significant, their clinical significance is debatable. The third study had the most impressive results, yet even those were very modest, lowering agitation by only five points compared to placebo (Lee D et al, JAMA Neurol 2023;80(12):1307–1316). On the 174-point Cohen-Mansfield Agitation Inventory scale used by the researchers, a drop of at least 17 points is considered clinically meaningful (de Mauleon A et al, Alzheimers Dement 2021;17(10):1687–1697).
Brexpiprazole has not been studied for psychosis related to Alzheimer’s disease, and its benefits in agitation were not due to improvements in paranoia or other psychotic symptoms, according to a post-hoc analysis (Behl S et al, Am J Geriatr Psychiatry 2024;32(4):S94). These trials only apply to agitation in Alzheimer’s disease. For other dementias, brexpiprazole’s anti-agitation effects are only supported by case reports. In dementia with Lewy bodies, antipsychotics are relatively contraindicated due to a high risk of neuroleptic malignant syndrome, delirium, and death.
Regarding safety, the trial results were reassuring, with elevated extrapyramidal symptoms but no increase in falls, fractures, cerebrovascular events, or QT prolongation (Marinheiro G et al, Neurol Sci 2024;45(10):4679–4686). The mortality rate was higher in the brexpiprazole groups (6 patients, 0.9%) than placebo (1 patient, 0.3%), but the investigators considered the deaths to be unrelated to brexpiprazole treatment.
Carlat Verdict: While it is FDA indicated for agitation in dementia, brexpiprazole still carries risks in this population, including excess mortality. Nonpharmacologic interventions remain first line for agitation in all types of dementia.
† Footnote: Olanzapine (5–15 mg) and risperidone (0.5–3 mg) have controlled trials, while aripiprazole (5–30 mg) and quetiapine (100–400 mg) have open-label data.
Newsletters
Please see our Terms and Conditions, Privacy Policy, Subscription Agreement, Use of Cookies, and Hardware/Software Requirements to view our website.
© 2026 Carlat Publishing, LLC and Affiliates, All Rights Reserved.


_-The-Breakthrough-Antipsychotic-That-Could-Change-Everything.webp?t=1729528747)



